Ph.D. | Thesis summary
Computational Modelling of Tissue Energy Interaction in Acoustic and Optical Imaging for
In situ Diagnostic Histopathology

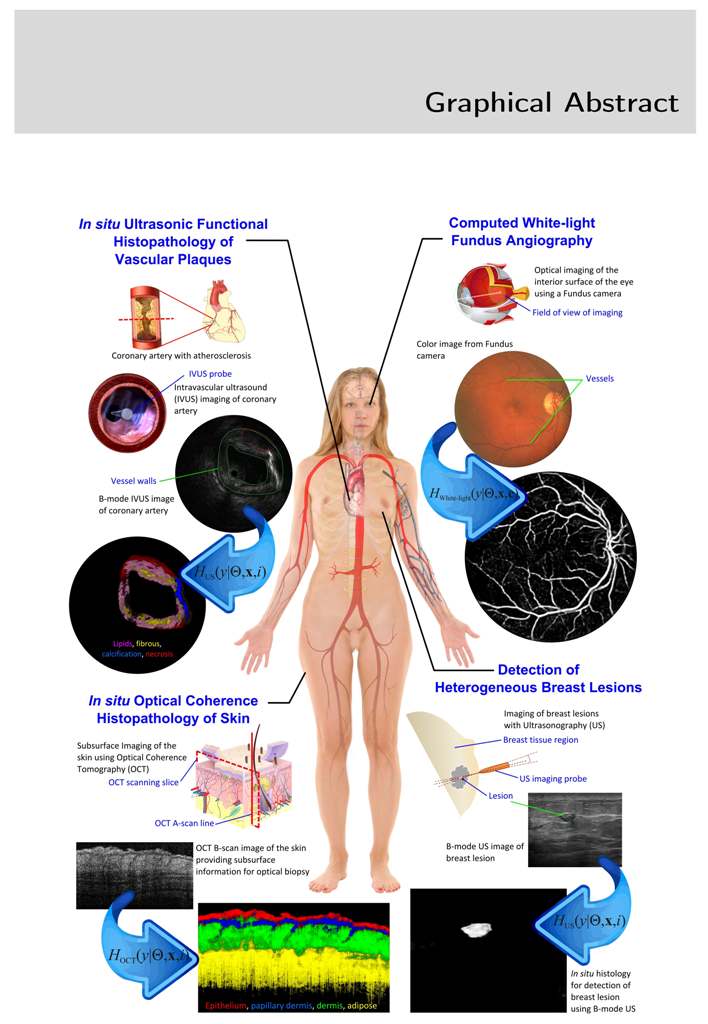
Soft tissues like skin, fat, muscles and blood vessels experience benign and malignant tumors, excess deposition of extracellular matrix, cellular hyperplasia and hypoplasia, and are medically termed as lesions. In diagnostics, a small quantity of tissue is collected from the lesion as biopsy or through aspiration, followed by evaluation by an expert Pathologist. This invasive procedure involves patient discomfort and results in 48-72 hours of delay in reporting. Moreover, this practice is not feasible for critical organs, eyes, coronary vessels and healing wounds.
In this thesis, a set of multidimensional signal and image processing algorithms are proposed and evaluated for in situ diagnostic histopathology in real time using non-destructive subsurface imaging. The rational was to develop an analytically converging solution to a set of statistical physics equations to model tissue-energy interaction in acoustic and optical imaging using a transfer learning framework.
In acoustic imaging ultrasonic propagation and backscattering in heterogeneous tissues are modelled and used to characterize tissues. In intravascular ultrasound (IVUS) a model for uncompressed signals identifies fibrous tissue, calcified, lipidic plaque and necrosis at sensitivity of 97%, 99%, 99% and 96% respectively with specificity above 80%. In B-mode ultrasonic imaging, the model identifies BIRADS Cat 2,3,4,5 lesions at 100%, 100%, 100% and 95% detection rates respectively with classification rates in area under ROC of 0.99, 0.99, 0.98 and 0.87.
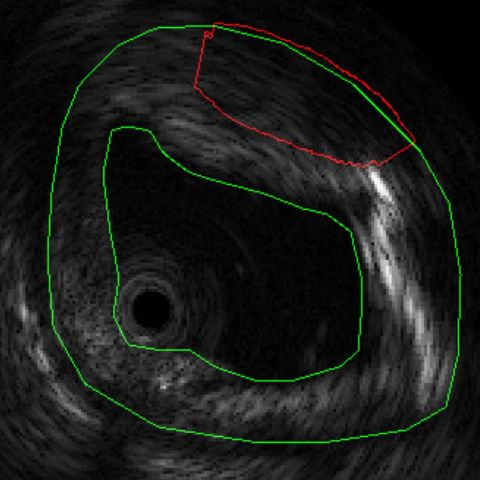
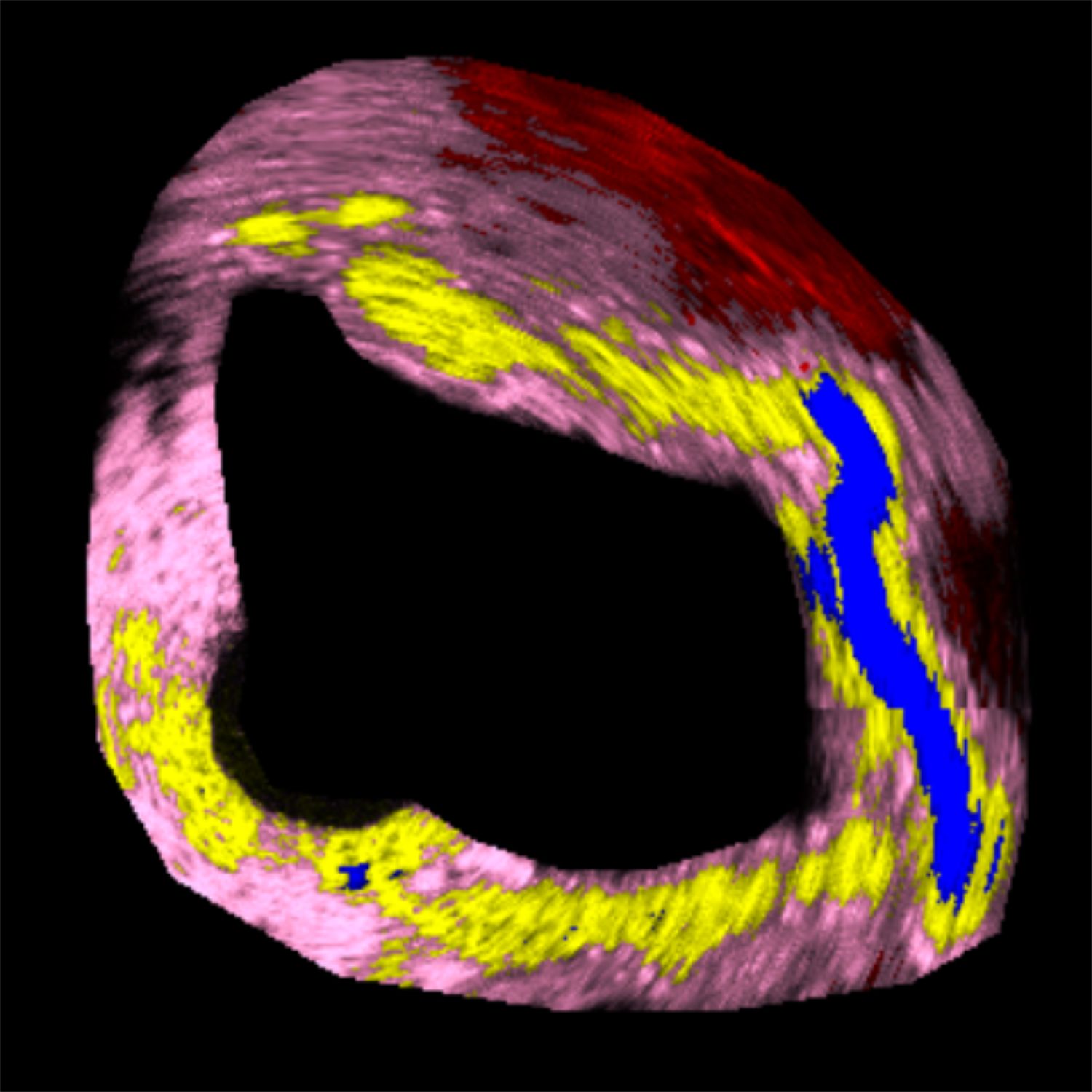
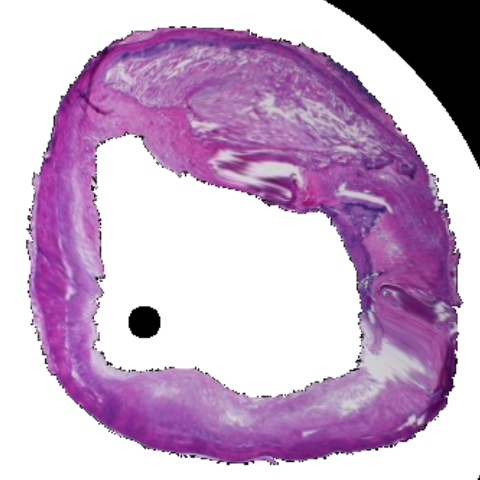
Figure 1: Tissue characterization in IVUS. (a) B-mode IVUS image of coronary artery with >40% stenosis. (b) Results of tissue characterization for in situ histology. Different tissues are color coded as Lipids, Fibrous, Calcification and Necrosis. (c) Conventional H&E stained histology of the corresponding section for comparison.
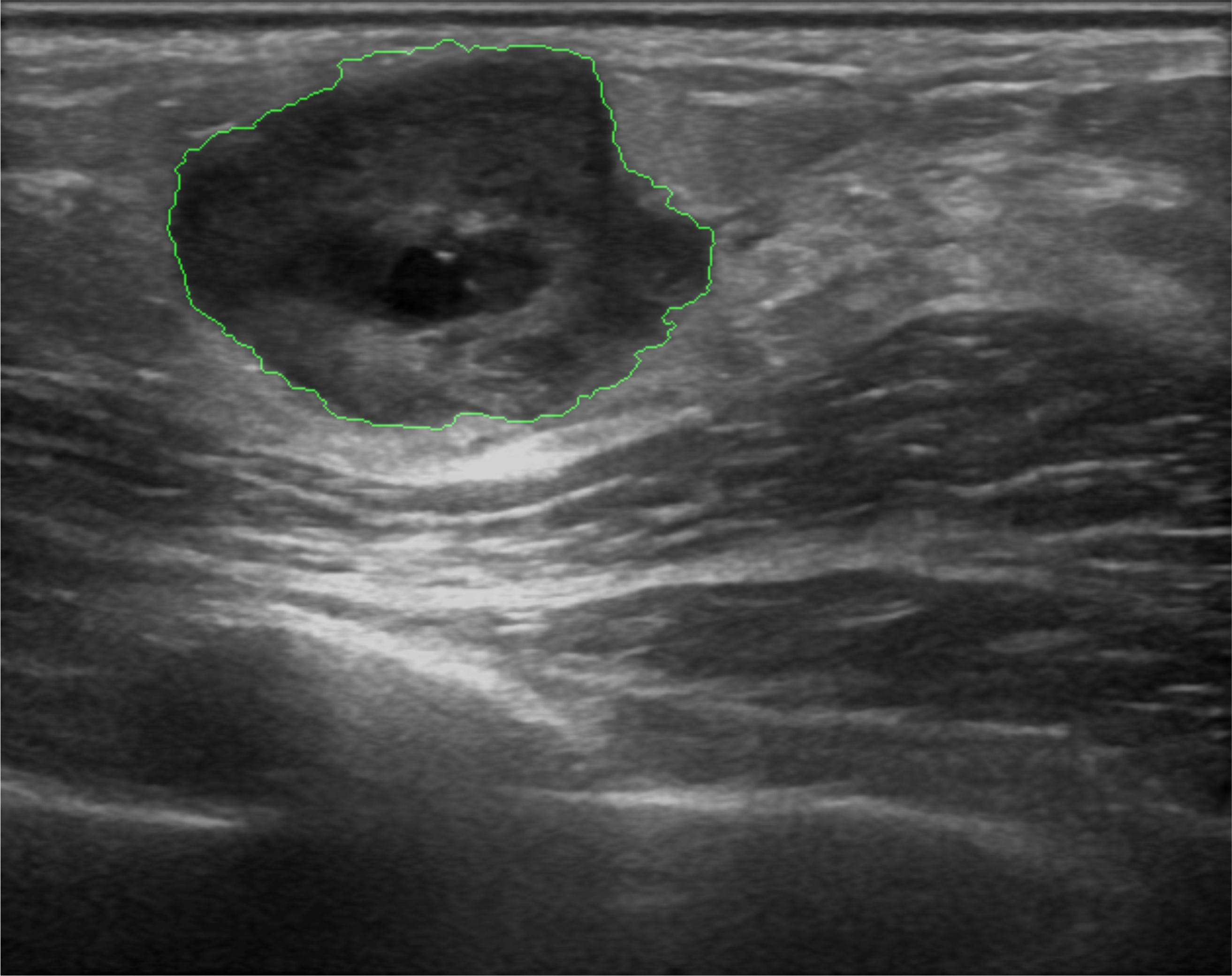
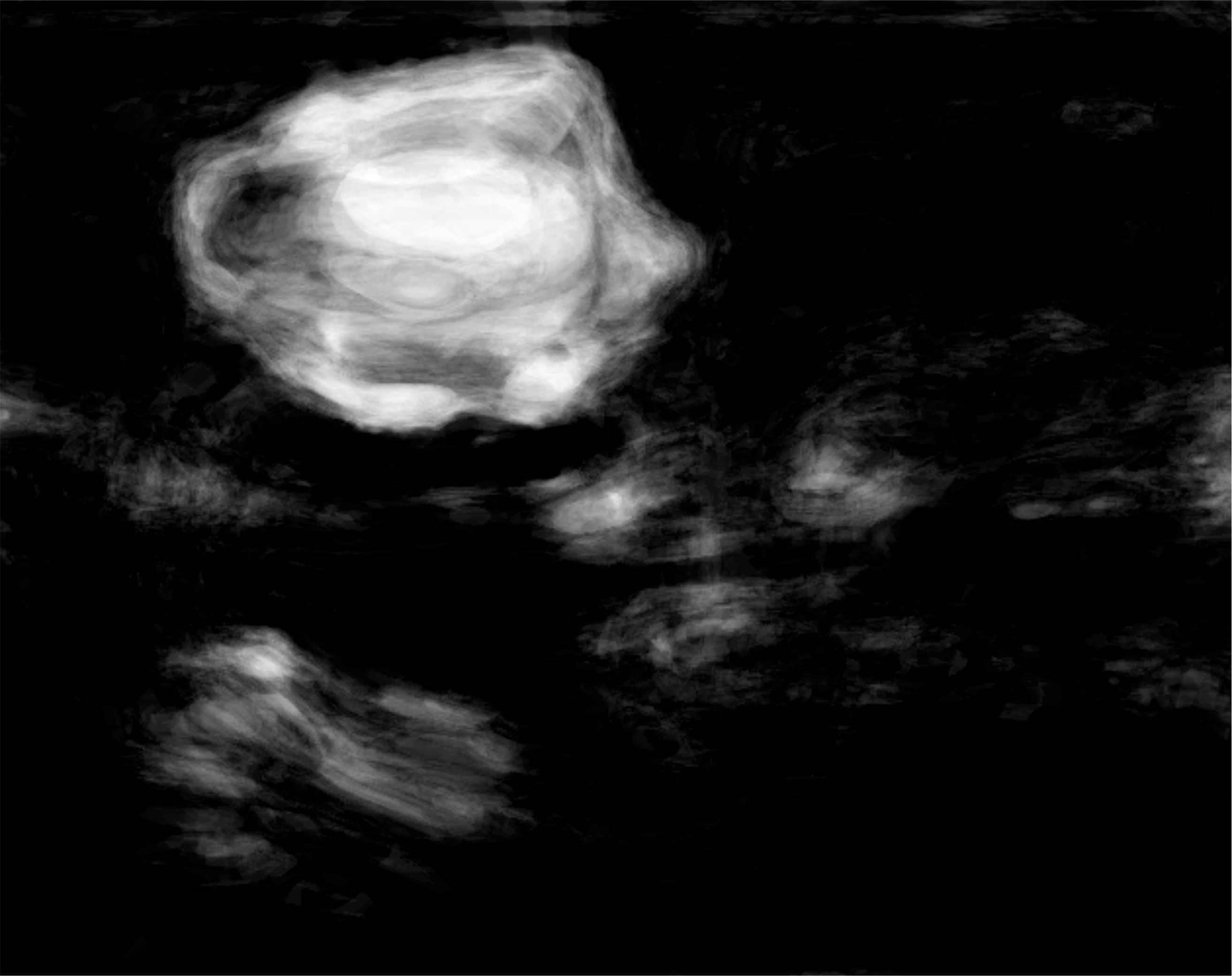
Figure 2: Heterogeneous lesion detection in Sonomammography. (a) B-mode US image of a breast lesions with heterogeneous echogenity and mixed posterior echo properties. Lesion boundary is marked in green. (b) Results of lesion detetion probability.
The photon-tissue interaction for non-ballistic and ballistic photons was modelled in ophthalmic imaging and optical coherence tomography (OCT). Retinal vessels are detected at an accuracy of 98% and ground truth consensus at kappa score of 0.83. Epidermis, papillary dermis, dermis and adipose tissue in healthy and wound tissue in mice skin was identified at sensitivity of 99%, 95%, 99% and 99% respectively using swept source OCT.
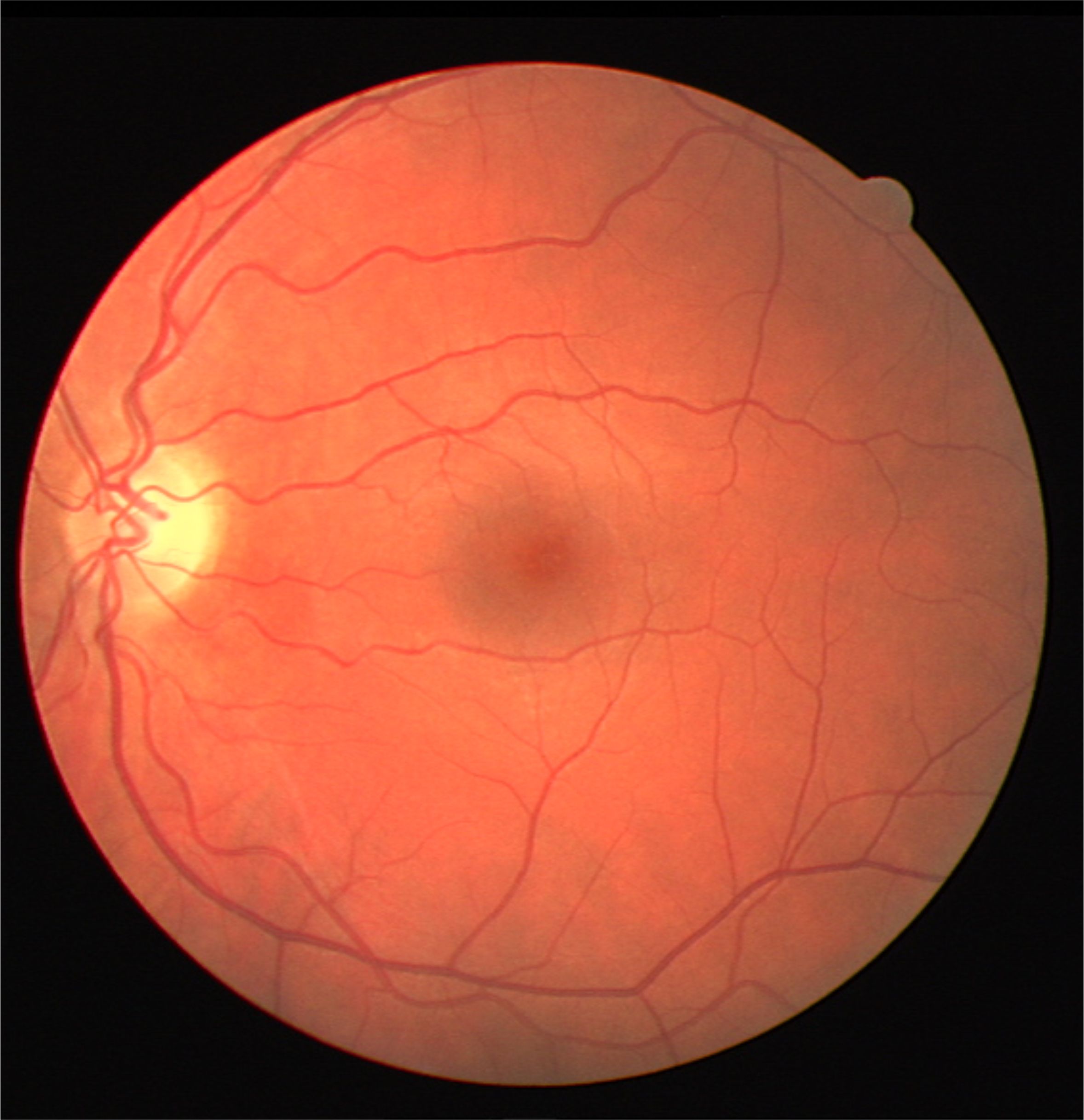
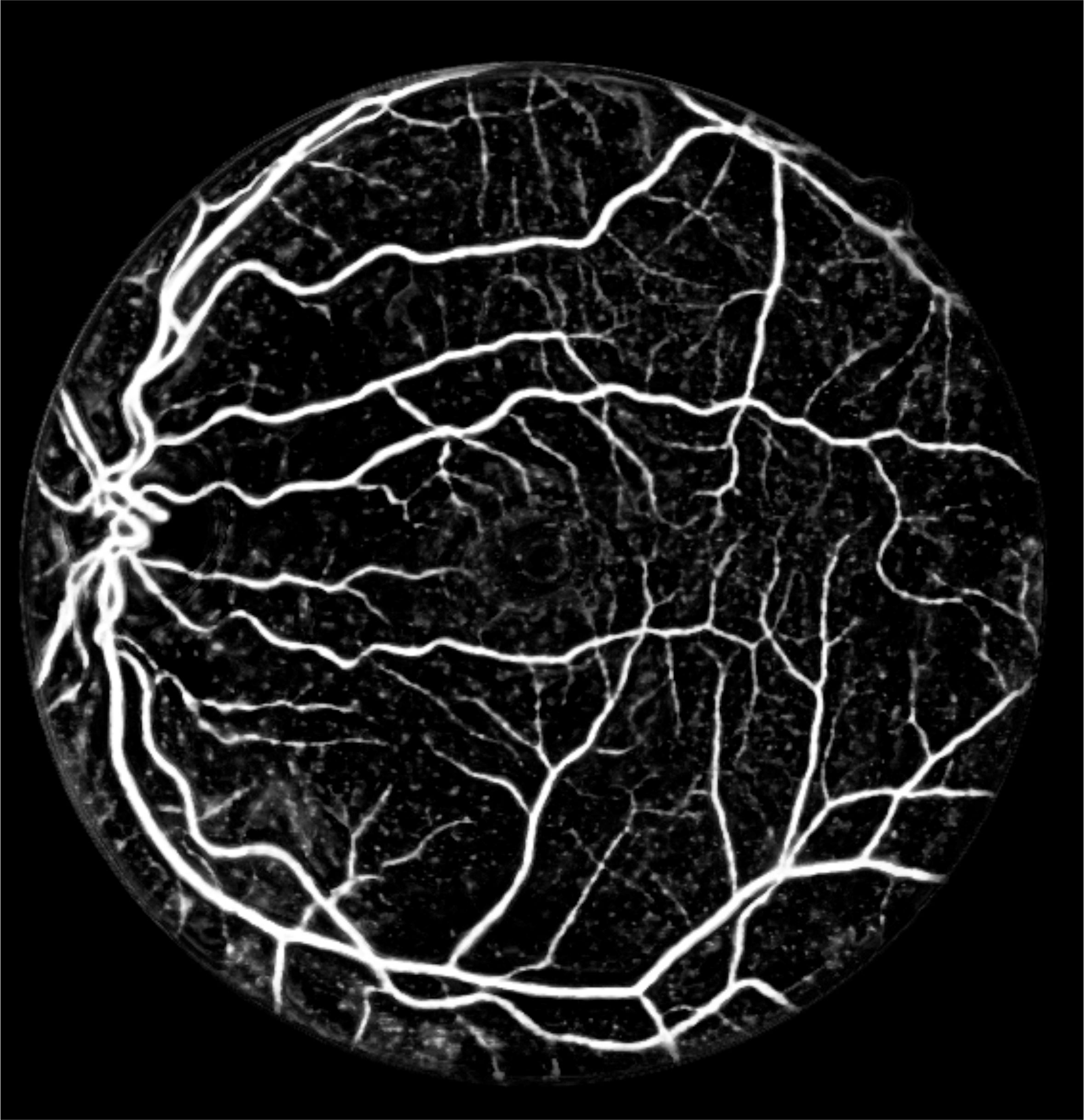
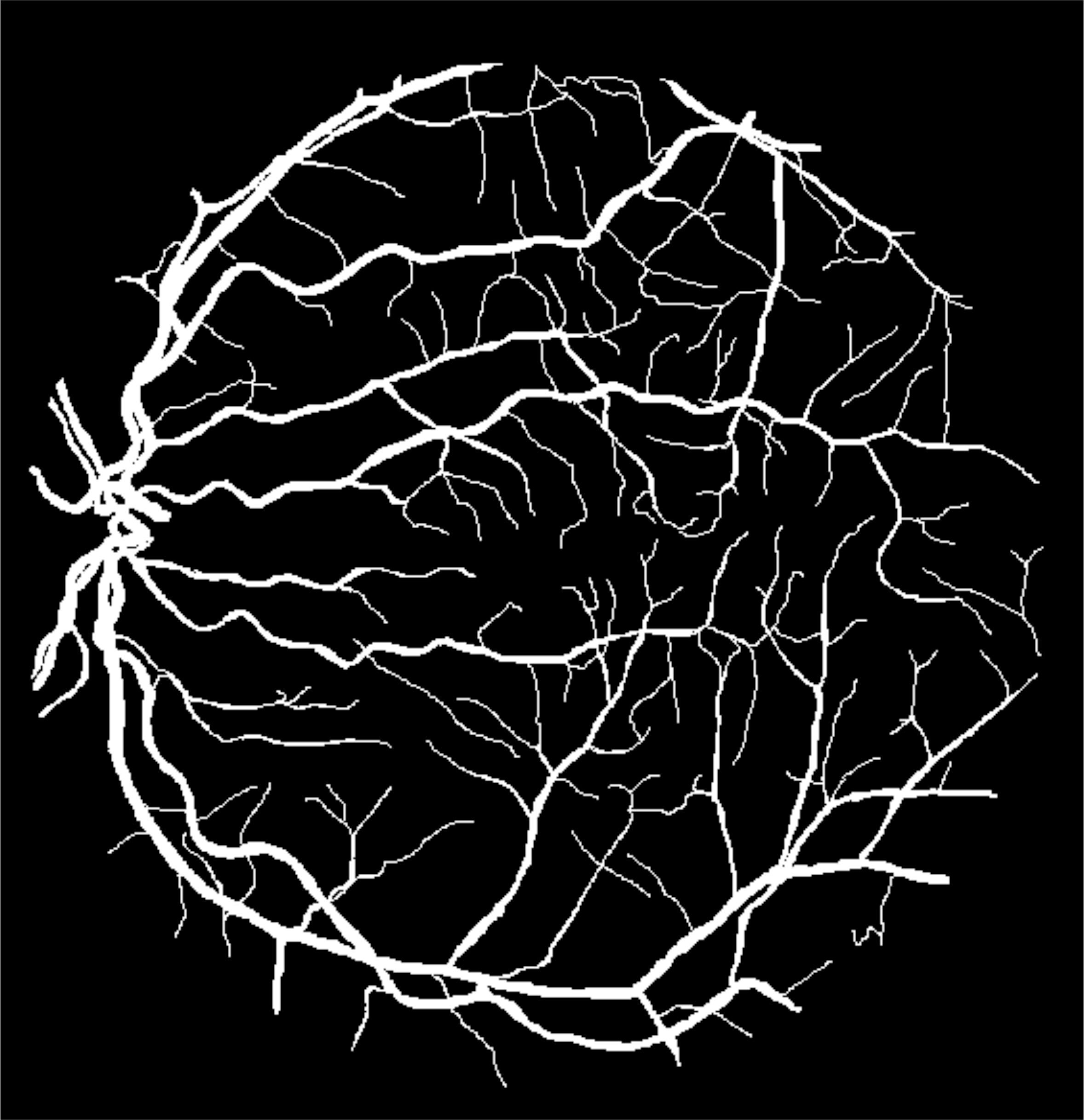
Figure 3: White light angiography solution in Fundus (Retinal) imaging. (a) Color fundus image of the retina acquired using a conventional ophthalmoscope in white light. (b) Detection blood vessels facilitating white-light angiography. (c) Ground truth location of vessels marked by an expert.
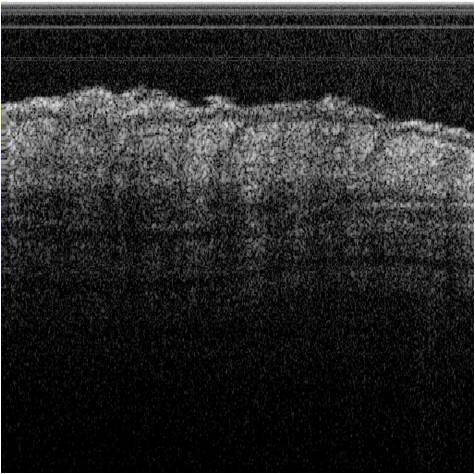
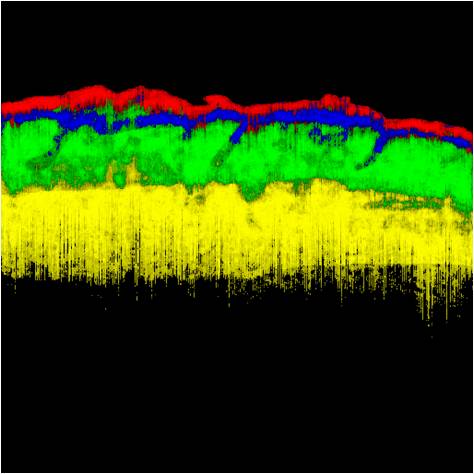
Figure 4: Tissue characterization in OCT. (a) Conventional intensity images of OCT of mice skin. (b) Results of tissue characterization for in situ histology. Different tissues are color coded as Epithelium, Papillary dermis, Dermis and Adipose tissue. (c) Conventional H&E stained histology of the corresponding section for comparison.